Previously, on “Lakirsal in the Lab” …
We began an experiment to test the pH level of Lakrisal (a.k.a. the worst candy in the world, IOHO). Lakrisal’s main ingredients are licorice extract, ammonium chloride and sugar, so we wanted to figure out the chemistry behind what made it so awful.
Our control group was a 5% ammonium chloride solution, which clocked in at about 5.13 on the pH scale. Then we diluted a crushed-up Lakrisal tablet in 100 ml of water and it came out looking like the stuff in the photo above. Which looks like … you can decide what it looks like. Not the most analytic or precise experiment, but the best one to figure out the pH scale given what we had.
Conclusion: our solution of Lakrisal …
… has a pH level of 5.92.
On the pH scale, that’s about here –
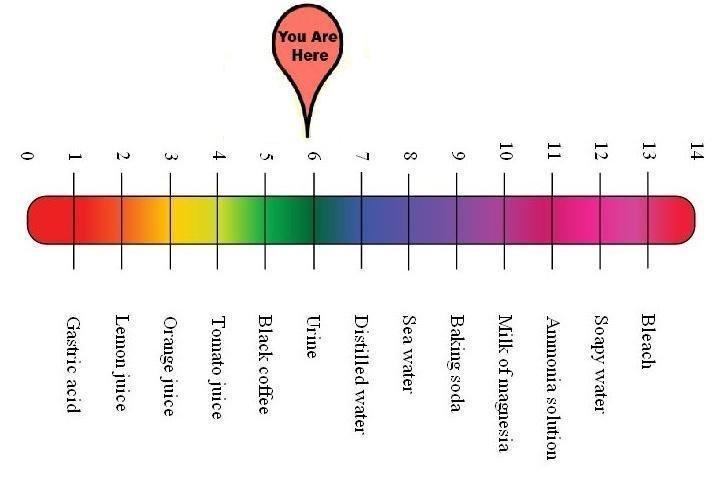
Still mildly acidic, like the ammonium chloride control group solution level of 5.13. But Lakrisal is a bit less acidic, and even closer to same pH level as urine! This candy just keeps getting better and better!
Even after all that, the brave Dr. Merrer wound up the courage to try the stuff (in tablet form, not liquid). She explained that that sour taste does come from the acidity in the tablet. “It’s pretty brutal.”












